Most of the water on Earth, 97% to be exact, is salt water found in the oceans. On the flip side, only 3% of the Earth’s water is fresh! 2% of the Earth’s water is in solid form, found in ice caps and glaciers. Because it is frozen and so far away, the fresh water in ice caps is not available for use by people or plants. That leaves about 1% of all the Earth’s water in a form usable to humans and land animals! This fresh water is found in lakes, rivers, streams, ponds, and in the ground.


In Wakefield, most of the students, rely on groundwater as a drinking supply for their households. Wells allow us to pull drinking water from groundwater sources, for everyday uses. In fact, 15% of Americans rely on their own private drinking water supplies.
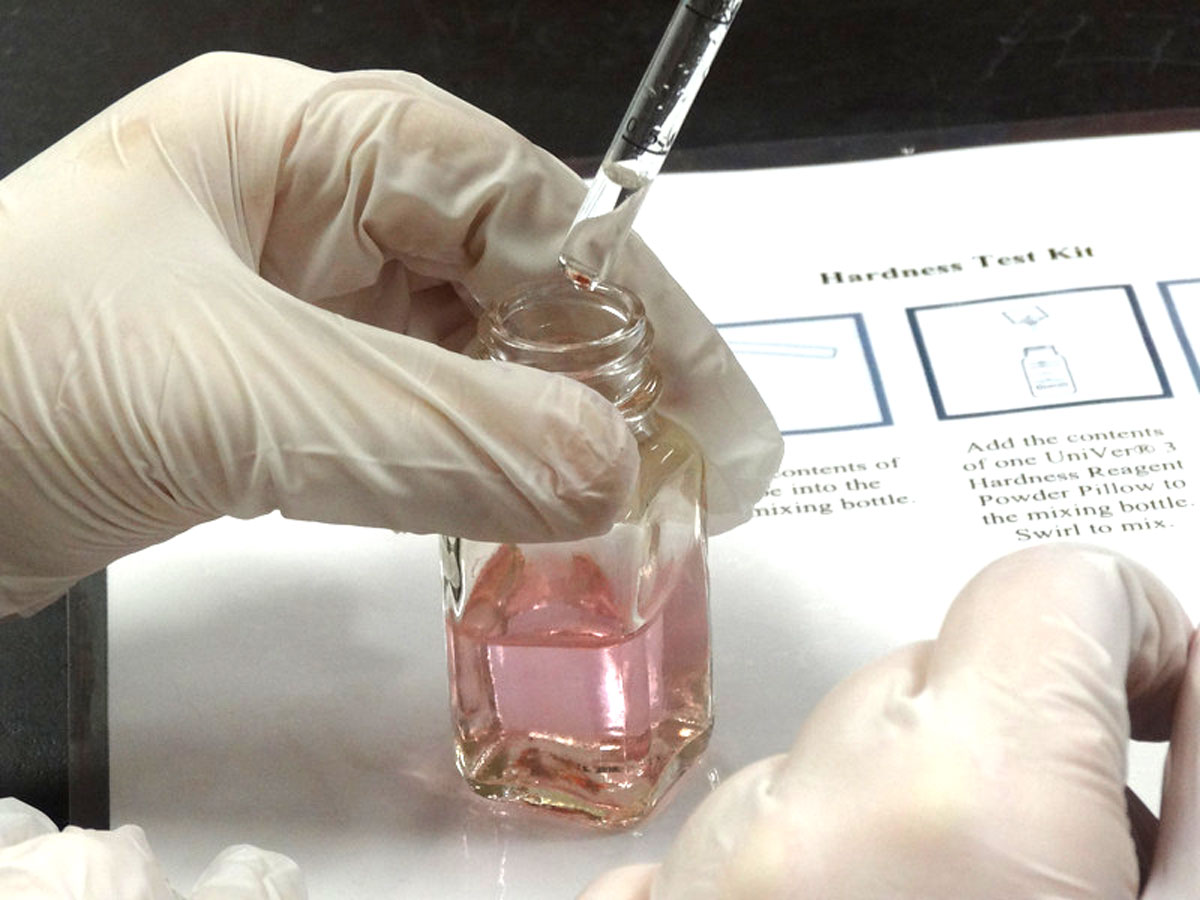
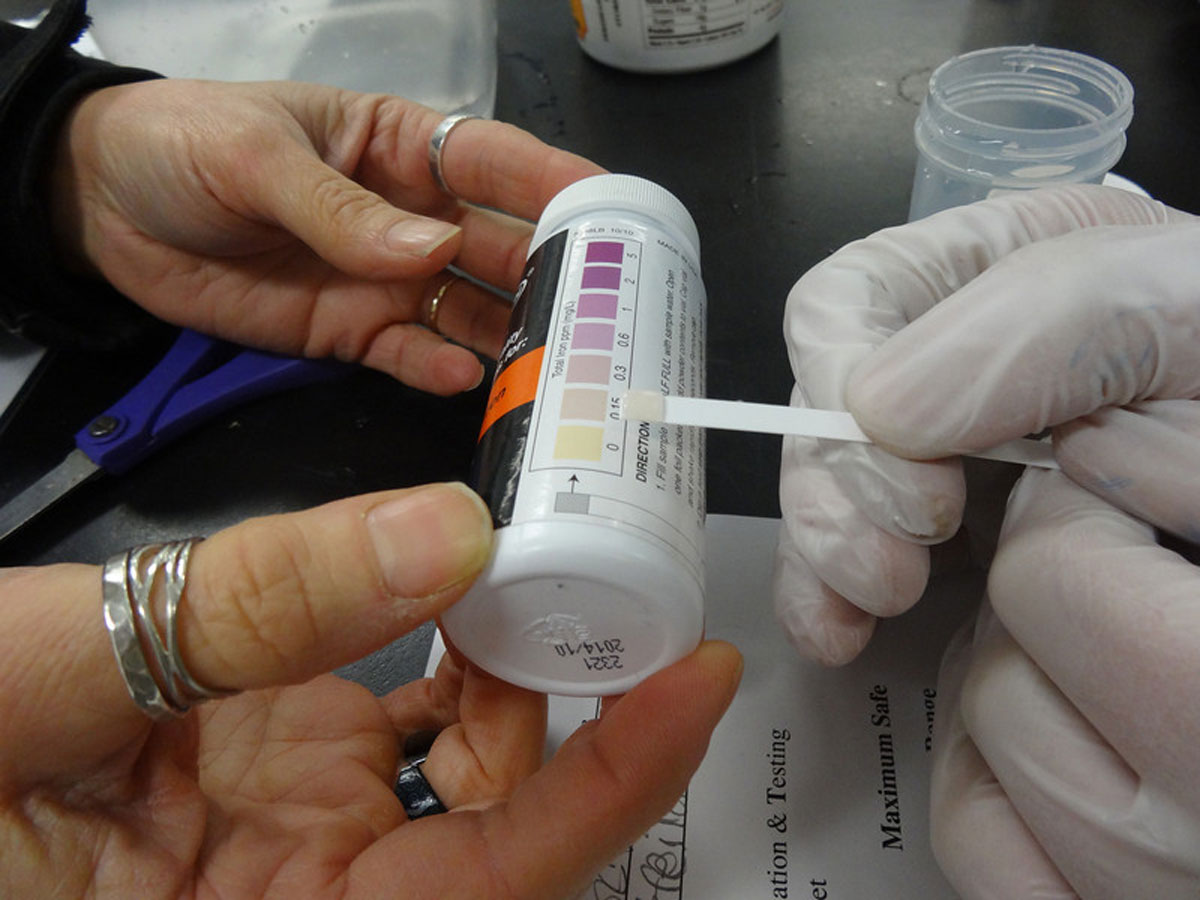
This week, the Paul School’s 7th grade participated in our well water testing module.
Students are given the opportunity to become scientists by performing chemical tests on water samples taken from their homes. The biggest concern for private drinking wells is contamination from industrial, agricultural, or household wastes.

The module begins with an introduction to groundwater, potential contamination sources, and how we determine what makes water safe to drink. Students were then given bottles to bring home with instructions on how to collect their samples. The following week, AWWA brought in volunteers to help guide the students through the testing stations.
Thank you to our amazing volunteers who helped make this day possible. Thanks to, Pete Dinger (Vice President of AWWA’s Board of Directors), Jeanne Achille (Secretary of AWWA’s Board of Directors), Mary Lenzen (AWWA board member), Rosemary Stewart (AWWA board member), Pastor Thomas (Middleton Gospel Chapel), and Maria (Paul School Volunteer).

The module concludes with a presentation of the testing results. The students are shown maps and graphs of their results, and a dialogue is started about why they may have gotten certain results. The students love comparing and contrasting their data to come up with hypotheses for why they got the results that they did.



Here are descriptions of the parameters the students tested:
Nitrates: They are the primary active ingredient in many fertilizers, and are colorless, odorless and tasteless.
pH: Measure of how acidic your water is. Low pH in water can leach toxic metals from plumbing systems into the water.
Conductivity: A measurement of the ions in a solution. High conductivity levels could be a sign of contamination from sewage or industrial waste.
Chloride: A key component of sodium chloride (salt). Roads can contribute as well as septic systems.
Iron: Iron can be a troublesome chemical in water supplies. Making up at least 5% of the earth’s crust, iron is one of the earth’s most plentiful resources. Iron related bacteria are responsible for the orange, rusty water spots often found on flatware. High levels could mean corroding pipes.
Hardness: The amount of dissolved Calcium (Ca) and Magnesium (Mg). Hard water will not “sud” and creates white deposits on pipes and fixtures.